With COVID cases rising this summer, what to know about the Paxlovid treatment
Published in News & Features
COVID-19 is rising in prevalence in many states nationally following the July 4 holiday week, but the role of the main therapy to treat it — the antiviral drug Paxlovid — is less clear.
Research studies over the past year found the drug less effective in real-world use than it was in clinical trials during the race in 2020 and 2021 to find treatments for COVID-19 and slow the global pandemic.
“Data from the original research studies conducted in 2021 among those with their first COVID-19 infection are not valid in extrapolating benefit today in 2025,” said Dr. David Boulware, an infectious disease specialist at the University of Minnesota. He added, “there likely is minimal benefit from antiviral therapy” for people who had previous infections or COVID vaccinations.
Prices for Pfizer’s drug remain elevated as well, though discount programs are widely available.
It is clear, however, COVID infections are rising, following the pattern of higher prevalence seen in prior summers. Testing of wastewater samples in Minnesota is showing another slight increase so far in levels of the coronavirus that causes COVID-19.
Holiday travel and family gatherings are believed to be a driver in the virus’ spread this time of year. National trends show rising and even high levels of COVID-19 in many southern and western states.
Only one in five Minnesotans are up to date with federal COVID vaccine recommendations.
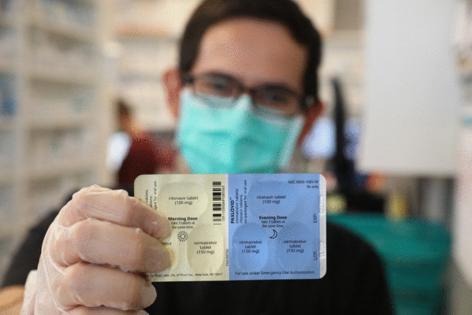
Paxlovid is an antiviral combination therapy that can reduce the risk of COVID-19 causing severe illness, hospitalization and death. The Food and Drug Administration authorized its emergency use in late 2021 during the pandemic and granted full approval in spring 2023.
Prescribed as a course of multiple pills per day, for five days, Paxlovid contains an antiviral drug, nirmatrelvir, that slows the spread of the coronavirus. It also contains ritonavir, which is commonly used to treat HIV but also increases the virus-fighting effectiveness of nirmatrelvir.
GoodRx estimates a five-day course of Paxlovid costs $1,400 or more. That amount raises concerns among health economists, even though Paxlovid is covered by most health insurance plans and eligible for discount programs operated by its manufacturer. An analysis by the Institute for Clinical and Economic Review in 2022 found that a price of $600 to $900 would make Paxlovid cost-effective, because it would have prevented at least some patients with COVID from enduring more expensive hospitalizations.
The federal government was paying $530 per course of Paxlovid in 2022, in part because it placed a bulk order of 24 million courses during the pandemic. However, the cost increased after the therapy received FDA approval and payment shifted to patients and health insurers.
This follows historical patterns in which drug companies charge higher prices for newly approved drugs to recoup the cost of researching and developing them. Harvard University researchers estimated in 2023 that Paxlovid costs $13 to produce.
Enthusiasm over Paxlovid waned when some studies found weaker-than-expected benefits, especially for people up to date on COVID-19 vaccines. UCLA researchers this winter found, at best, modest reductions in COVID hospitalizations among vaccinated adults, despite earlier clinical trial data that found a 90% reduction in COVID-related hospitalizations and deaths.
A follow-up study in 2022 by the CDC and Verona, Wis.-based Epic Research found a 51% reduction in hospitalizations, regardless of whether people had been vaccinated or previously infected. The CDC continues to recommend Paxlovid for treating mild-to-moderate COVID in adults who are 50 and older, unvaccinated, or have health conditions that put them at elevated risk from infections. The drug is urged for people with weakened immune systems.
Timing remains critical. Paxlovid must be taken within five days of symptom onset to work. Some studies have found more benefits if taken sooner. The oral medication remains broadly available at pharmacies via prescription, in part because of declining demand. Pfizer continues to operate a variety of discount plans, including the PAXCESS program that can reduce some or all costs of the medication for privately insured individuals. The Patient Assistance Program remains available through 2028, according to Pfizer, and will provide no-cost access to individuals with public insurance plans who cannot afford their copays and meet low-income guidelines.
_____
©2025 The Minnesota Star Tribune. Visit startribune.com. Distributed by Tribune Content Agency, LLC






Comments